Significance of Suppression of Cerebral Gluconeogenesis in the Protective Value of Exercise Post Conditioning Towards Ischemic Injury in Rats
Abstract
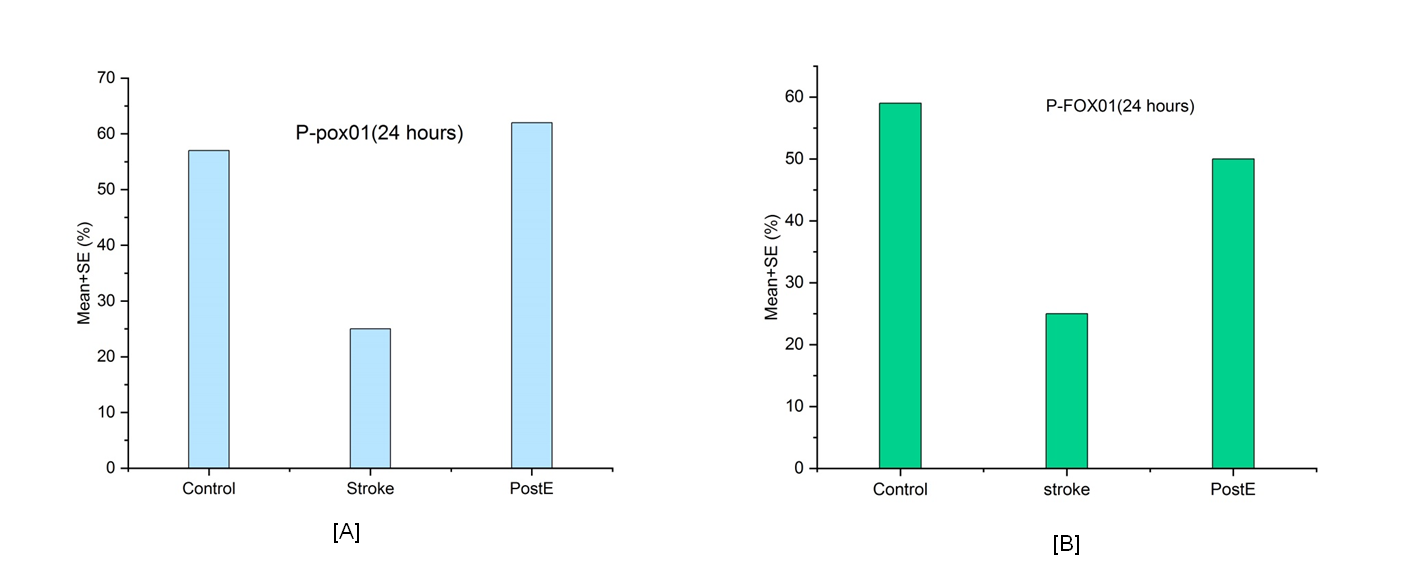
The practice of exercise before a stroke lowers neurovascular damage and improves functional results. In order to determine the extent to which regulation of gluconeogenesis was related to how much brain damage post-stroke exercise training (PostE) prevented, this research set out to investigate these questions. Middle Cerebral Artery (MCA) blockage for 72 hours ensued by 24 hours of resuscitation in young rats. After 24 hours after a reperfusion PostE's treadmill exercise was started. Infarct quantity, neurological deficits at one and three days after reperfusion as well as apoptosis of cells was used to gauge the extent of the brain injury. Oxaloacetate (OAA), glucose, lactate, pyretic acid, phosphoenolpyruvate (PEP), and ROS were quantified by ELISA, and immunofluorescence was used to the critical enzyme phosphoenolpyruvate carboxykinase (PCK)-1/2's site and production. By using Western blot, we were able to identify upstream pathways such as forehead 3-kinase (PI3K)/Akt, p-PI3K/Akt, transcription factor (FoxO1), and all of them. Additionally, immunofluorescence was used to find p-FoxO1 expressed in the cytoplasm. PostE is superior to non-exercise control. Subsequently one and three days, there were smaller brain infarction sizes, neurological problems, and cell death. On both days, OAA was higher in postE groups and lower affects the expression of tissue PCKs, PEP, pyretic acid, lactate, ROS, glucose. The workout environment also drastically reduced PCK-1/2 expressions. Additionally, PostE dramatically increased the expression of phosphorylated PI3K, AKT, and FoxO1 proteins at one and three days. In this work, PostE suppressed gluconeogenesis and decreased brain damage after stroke in conjunction with increased PI3K/AKT/FoxO1 signalling. These findings imply that FoxO1 control of gluconeogenesis is a factor in post-stroke brain protection.
References
Guo, S., Wehbe, A., Syed, S., Wills, M., Guan, L., Lv, S., Li, F., Geng, X. and Ding, Y., 2023. Cerebral Glucose Metabolism and Potential Effects on Endoplasmic Reticulum Stress in Stroke. Aging and Disease, 14(2), p.450.
Leech, T., Chattipakorn, N. and Chattipakorn, S.C., 2019. The beneficial roles of metformin on the brain with cerebral ischaemia/reperfusion injury. Pharmacological research, 146, p.104261.
Liang, J., Han, R. and Zhou, B., 2021. Metabolic reprogramming: strategy for ischemic stroke treatment by ischemic preconditioning. Biology, 10(5), p.424.
Oyelaja-Akinsipo, O.B., Dare, E.O. and Katare, D.P., 2020. Protective role of diosgenin against hyperglycaemia-mediated cerebral ischemic brain injury in zebrafish model of type II diabetes mellitus. Heliyon, 6(1), p.e03296.
Guan, L., Lee, H., Geng, X., Li, F., Shen, J., Ji, Y., Peng, C., Du, H. and Ding, Y., 2022. Neuroprotective Effects of Pharmacological Hypothermia on Hyperglycolysis and Gluconeogenesis in Rats after Ischemic Stroke. Biomolecules, 12(6), p.851.
Luo, Y., Chen, P., Yang, L. and Duan, X., 2023. Metabolomic analysis and pharmacological validation of the cerebral protective effect of 3, 4 dihydroxybenzaldehyde on cerebral ischemia reperfusion injury. Molecular Medicine Reports, 27(1), pp.1-15.
Qu, Y., Cao, J., Wang, D., Wang, S., Li, Y. and Zhu, Y., 2022. 14, 15-Epoxyeicosatrienoic Acid Protect Against Glucose Deprivation and Reperfusion-Induced Cerebral Microvascular Endothelial Cells Injury by Modulating Mitochondrial Autophagy via SIRT1/FOXO3a Signaling Pathway and TSPO Protein. Frontiers in Cellular Neuroscience, 16.
Li, F., Geng, X., Ilagan, R., Bai, S., Chen, Y. and Ding, Y., 2023. Exercise postconditioning reduces ischemic injury via suppression of cerebral gluconeogenesis in rats. Brain and Behavior, 13(1), p.e2805.
Miao, W., Yan, Y., Bao, T.H., Jia, W.J., Yang, F., Wang, Y., Zhu, Y.H., Yin, M. and Han, J.H., 2020. Ischemic postconditioning exerts neuroprotective effect through negatively regulating PI3K/Akt2 signaling pathway by microRNA-124. Biomedicine & Pharmacotherapy, 126, p.109786.
Wang, Q., Zuurbier, C.J., Huhn, R., Torregroza, C., Hollmann, M.W., Preckel, B., van den Brom, C.E. and Weber, N.C., 2023. Pharmacological Cardioprotection against Ischemia Reperfusion Injury—The Search for a Clinical Effective Therapy. Cells, 12(10), p.1432.
Elsaid, F.H., Khalil, A.A., Ibrahim, E.M., Mansour, A. and Hussein, A.M., 2019. Effects of exercise and stevia on renal ischemia/reperfusion injury in rats. Acta scientiarum polonorum. Technologia alimentaria, 18(3).
Cao, C., Liu, H.M., Li, W., Wu, Y., Leng, Y., Xue, R., Chen, R., Tang, L.H., Sun, Q., Xia, Z. and Tang, Q.Z., 2020. Role of adiponectin in diabetes myocardial ischemia-reperfusion injury and ischemic postconditioning. Acta Cirúrgica Brasileira, 35.
Wang, J., Zhang, W. and Wu, G., 2021. Intestinal ischemic reperfusion injury: Recommended rats model and comprehensive review for protective strategies. Biomedicine & Pharmacotherapy, 138, p.111482.
Danková, M., Domoráková, I., Fagová, Z., Stebnický, M. and Mechírová, E., 2021. Induction of ischemic tolerance by remote perconditioning or postconditioning as neuroprotective strategy for spinal cord motor neurons. Life Sciences, 283, p.119789.
Eskaf, J., Cleveland, W.J. and Riess, M.L., 2021. No Direct Postconditioning Effect of Poloxamer 188 on Mitochondrial Function after Ischemia Reperfusion Injury in Rat Isolated Hearts. International Journal of Molecular Sciences, 22(9), p.4879.
Boulghobra, D., Coste, F., Geny, B. and Reboul, C., 2020. Exercise training protects the heart against ischemia-reperfusion injury: A central role for mitochondria?. Free Radical Biology and Medicine, 152, pp.395-410.
Miao, W., Yan, Y., Bao, T.H., Jia, W.J., Yang, F., Wang, Y., Zhu, Y.H., Yin, M. and Han, J.H., 2020. Ischemic postconditioning exerts neuroprotective effect through negatively regulating PI3K/Akt2 signaling pathway by microRNA-124. Biomedicine & Pharmacotherapy, 126, p.109786.
Crisafulli, A., Pagliaro, P., Roberto, S., Cugusi, L., Mercuro, G., Lazou, A., Beauloye, C., Bertrand, L., Hausenloy, D.J., Aragno, M. and Penna, C., 2020. Diabetic cardiomyopathy and ischemic heart disease: prevention and therapy by exercise and conditioning. International journal of molecular sciences, 21(8), p.2896.
Seara, F.A., Olivares, E.L. and Nascimento, J.H., 2020. Anabolic steroid excess and myocardial infarction: From ischemia to reperfusion injury. Steroids, 161, p.108660.
Fan, Y., Wang, Y., Ji, W., Liu, K. and Wu, H., 2021. Exercise preconditioning ameliorates cognitive impairment and anxiety-like behavior via regulation of dopamine in ischemia rats. Physiology & Behavior, 233, p.113353.
Gerstmeier, J., Kretzer, C., Di Micco, S., Miek, L., Butschek, H., Cantone, V., Bilancia, R., Rizza, R., Troisi, F., Cardullo, N. and Tringali, C., 2019. Novel benzoxanthene lignans that favorably modulate lipid mediator biosynthesis: A promising pharmacological strategy for anti-inflammatory therapy. Biochemical Pharmacology, 165, pp.263-274.
Aykan, D.A., Yıldız, B.T., Kazancı, Ü., Seyithanoğlu, M., Koca, T. and Ural, A., 2020. Immunosuppressants and Ischemic Postconditioning in the Management of Brain Ischemia in Rats: The Role of Pharmacologic and Nonpharmacologic Treatments. Eur J Ther, 26(1), pp.53-60.
Zheng, Y., Wan, G., Yang, B., Gu, X. and Lin, J., 2020. Cardioprotective natural compound pinocembrin attenuates acute ischemic myocardial injury via enhancing glycolysis. Oxidative Medicine and Cellular Longevity, 2020.
Thorens, B., 2022. Neuronal regulation of glucagon secretion and gluconeogenesis. Journal of Diabetes Investigation, 13(4), pp.599-607.
Guo, H., Yin, H., Zuo, Z., Yang, Z., Yang, Y., Wei, L., Cui, H., Deng, H., Chen, X., Chen, J. and Zhu, Y., 2021. Oxidative stress-mediated apoptosis and autophagy involved in Ni-induced nephrotoxicity in the mice. Ecotoxicology and Environmental Safety, 228, p.112954.
Otsuka, S., Sakakima, H., Tani, A., Nakanishi, K., Takada, S., Norimatsu, K., Maejima, H. and Maruyama, I., 2021. Effects of detraining on preconditioning exercise-induced neuroprotective potential after ischemic stroke in rats. Brain Structure and Function, 226(7), pp.2169-2180.
Castrén, E. and Monteggia, L.M., 2021. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biological psychiatry, 90(2), pp.128-136.
Mason, I.C., Qian, J., Adler, G.K. and Scheer, F.A., 2020. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia, 63(3), pp.462-472.
Geng, X., Shen, J., Li, F., Yip, J., Guan, L., Rajah, G., Peng, C., DeGracia, D. and Ding, Y., 2021. Phosphoenolpyruvate carboxykinase (PCK) in the brain gluconeogenic pathway contributes to oxidative and lactic injury after stroke. Molecular Neurobiology, 58, pp.2309-2321.
Bian, X., Jiang, H., Meng, Y., Li, Y.P., Fang, J. and Lu, Z., 2022. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends in Cell Biology.